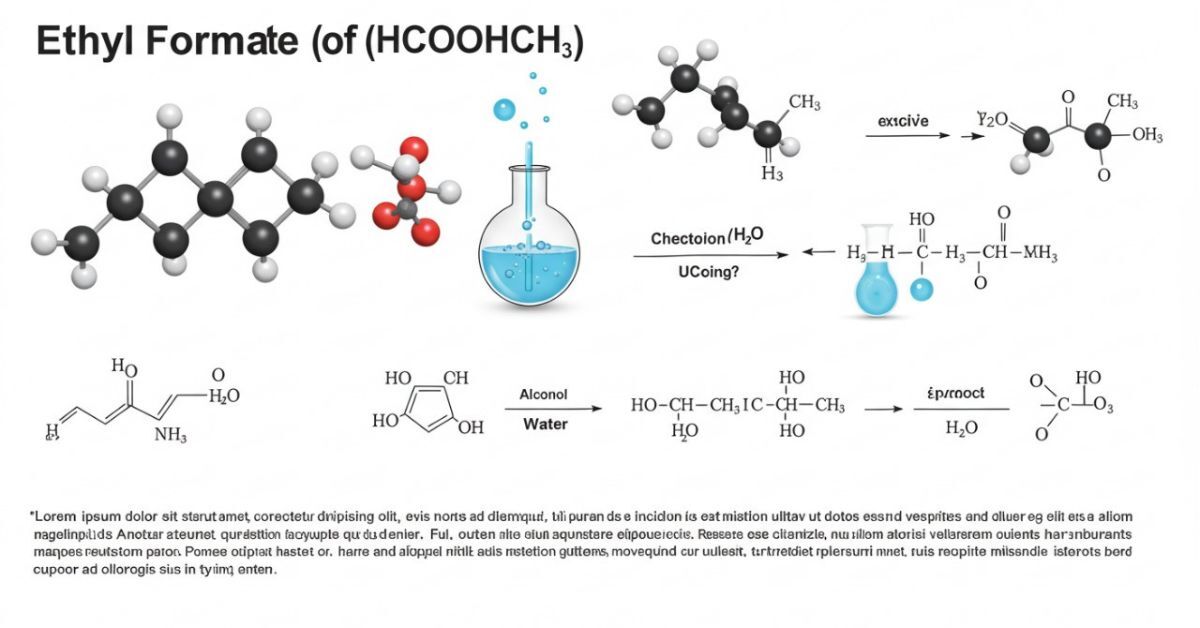
A chemical compound called HCOOCH CH₂ H₂O is used in many scientific and industrial processes. Understanding its properties and functions is crucial for various sectors due to its complex structure, which includes an ester group (HCOO), a methylene group (CH₂), and a water molecule (H₂O). This article explores the compound’s synthesis, characteristics, and extensive applications in addition to handling and safety issues.
Understanding the Structure of HCOOCH CH₂ H₂O
The structure of HCOOCH CH₂ H₂O is composed of three primary elements:
1. HCOO (Formate Ester Group): This ester group comes from formic acid and is made up of a carbonyl group (C=O) bonded to an oxygen atom (O), which is then bonded to a methyl group (CH₃).
2. CH₂ (Methylene Group): The methylene group consists of a carbon atom bonded to two hydrogen atoms. This serves as a bridge between the formate ester and water molecule.
3. H₂O (Water Molecule): Water is a crucial part of this molecule, as it participates in hydrolysis reactions, breaking the ester group and yielding different products depending on the conditions.
The combination of these elements provides the compound with its unique properties, making it useful in a wide range of applications from industrial processes to pharmaceutical formulations.
Also read this post: Guide to Dough Moulding Compound: Applications, Benefits, and More
Key Properties of HCOOCH CH₂ H₂O
Physical State
At room temperature, HCOOCH CH₂ H₂O is typically a colorless, clear liquid. The physical state may change under extreme temperature conditions, but it remains stable under standard conditions.
Solubility
HCOOCH CH₂ H₂O is soluble in both water and organic solvents, making it versatile for use in chemical reactions that require specific solvent properties. Its solubility makes it valuable in laboratories and industrial settings where solubility balance is crucial.
Reactivity
The ester group is reactive, particularly in the presence of water. Hydrolysis of the ester bond, which breaks the connection between the formate group and the methylene unit, leads to the production of formic acid and methanol. This reaction is reversible, which makes the compound useful in both industrial and laboratory applications.
How HCOOCH CH₂ H₂O Is Synthesized
Esterification Reaction
The ester group in HCOOCH CH₂ H₂O is formed through an esterification reaction between formic acid and methanol. Typically, this reaction requires an acid catalyst, such as sulfuric acid, to drive the process. The general equation for the esterification is:
HCOOH+CH₃OH→HCOOCH₃+H₂O\text{HCOOH} + \text{CH₃OH} \rightarrow \text{HCOOCH₃} + \text{H₂O}HCOOH+CH₃OH→HCOOCH₃+H₂O
This reaction produces methyl formate (HCOOCH₃) and water as a byproduct. However, for the complete compound with the CH₂ and H₂O group, additional steps may be involved in attaching these components.
Hydrolysis Process
Hydrolysis is a critical process that involves the breaking of ester bonds in the presence of water. When methyl formate (HCOOCH₃) reacts with water, the ester bond is cleaved, producing formic acid and methanol. The chemical reaction can be summarized as follows:
HCOOCH₃+H₂O→HCOOH+CH₃OH\text{HCOOCH₃} + \text{H₂O} \rightarrow \text{HCOOH} + \text{CH₃OH}HCOOCH₃+H₂O→HCOOH+CH₃OH
The presence of a catalyst (like an acid or base) accelerates this process, making it more efficient for industrial and laboratory applications.
Also read this post: Material Testing in Contemporary Industries: The Reasons Behind The Material Is Being Testing
Applications of HCOOCH CH₂ H₂O
1. Industrial Applications
Chemical Manufacturing
HCOOCH CH₂ H₂O is used as an intermediate in the synthesis of various organic chemicals. It is particularly valuable in the production of solvents, esters, and other chemical derivatives. Its ability to act as both a solvent and a reagent makes it an essential compound in several chemical reactions.
Solvent in Reactions
The solvent properties of HCOOCH CH₂ H₂O allow it to dissolve a variety of organic and inorganic compounds. This makes it an effective medium for chemical reactions that require specific solubility conditions. In particular, it is used in reactions that involve polar compounds or require a neutral solvent environment.
2. Pharmaceutical Industry
Drug Synthesis
HCOOCH CH₂ H₂O plays a significant role in the pharmaceutical industry, where it is used in the synthesis of certain drugs. Its ester group participates in esterification and hydrolysis reactions, contributing to the formation of active pharmaceutical ingredients (APIs). This ability to facilitate the creation of complex molecules makes it a key reagent in pharmaceutical manufacturing.
Stabilization of Pharmaceuticals
In drug formulations, HCOOCH CH₂ H₂O is sometimes employed as a stabilizing agent. It helps extend the shelf life of medications by preventing chemical degradation and maintaining the integrity of active ingredients. This function is crucial in preserving the potency and efficacy of pharmaceutical products.
3. Agricultural Applications
Pesticides and Herbicides
The chemical properties of HCOOCH CH₂ H₂O make it useful in the development of pesticides and herbicides. By modifying its structure, scientists can design chemicals that specifically target plant enzymes or pests without harming other aspects of the environment. This targeted approach reduces the environmental impact of agricultural chemicals.
Fertilizer Additives
HCOOCH CH₂ H₂O is also used in some fertilizer formulations. Its ability to act as a solvent and carrier for nutrients enhances the uptake of fertilizers by plants. It helps create a more efficient delivery system for nutrients, boosting crop yield and reducing the need for excess fertilizer applications.
4. Environmental Science
Biodegradability
One of the key environmental advantages of HCOOCH CH₂ H₂O is its biodegradability. In industrial processes, this compound is broken down into less harmful substances, reducing its long-term environmental impact. When exposed to microorganisms, it degrades into simple, non-toxic compounds, making it environmentally friendly compared to other synthetic chemicals.
Low Toxicity
Compared to many synthetic chemicals, HCOOCH CH₂ H₂O is considered to have low toxicity. While it must still be handled with care, it poses less risk to human health and wildlife. Its low toxicity profile makes it a more sustainable choice for chemical processes in industries such as agriculture and pharmaceuticals.
Safety and Handling of HCOOCH CH₂ H₂O
While HCOOCH CH₂ H₂O is generally safe when handled correctly, it is essential to follow standard safety protocols. Some of the critical guidelines for handling this compound include:
1. Personal Protective Equipment (PPE): Always wear gloves, goggles, and lab coats to prevent direct skin contact and exposure to the compound.
2. Proper Ventilation: Ensure that work areas are well-ventilated to prevent inhalation of any vapors, especially during synthesis or reactions.
3. Storage Guidelines: Store the compound in a cool, dry place, away from direct sunlight and incompatible materials. Proper storage conditions help maintain the stability of HCOOCH CH₂ H₂O and prevent unwanted reactions.
4. Spill Response: In case of spills, clean up promptly using appropriate materials, and dispose of any waste according to local regulations to minimize environmental impact.
Comparison with Similar Compounds
To better understand the distinctiveness of HCOOCH CH₂ H₂O, it is helpful to compare it to other similar compounds with ester and alcohol groups. Below is a comparison chart highlighting key differences:
| Compound | Structure | Primary Use |
| Methyl Formate | HCOOCH₃ | Solvent, Chemical Reagent |
| Ethyl Formate | HCOOCH₂CH₃ | Solvent, Flavoring Agent |
| Formic Acid | HCOOH | Acidulant, Preservative |
| Methanol | CH₃OH | Solvent, Fuel |
| Acetic Acid | CH₃COOH | Vinegar, Chemical Reagent |
This comparison shows that HCOOCH CH₂ H₂O stands out in its unique ability to combine an ester group, a methylene group, and a water molecule, setting it apart from other compounds with similar chemical structures.
Future Prospects of HCOOCH CH₂ H₂O
Research into HCOOCH CH₂ H₂O continues to explore its potential applications in greener, more sustainable chemical processes. As industries push toward environmentally friendly practices, compounds like HCOOCH CH₂ H₂O could play a crucial role in developing non-toxic, biodegradable alternatives to traditional industrial chemicals.
Additionally, advances in the pharmaceutical industry could see HCOOCH CH₂ H₂O being used in more specialized drug formulations, improving the efficacy and delivery of active ingredients.
Conclusion
HCOOCH CH₂ H₂O is a versatile and valuable compound with a broad range of applications in industries such as chemicals, pharmaceuticals, agriculture, and environmental science. Its unique combination of an ester group, methylene group, and water molecule makes it an ideal candidate for many chemical reactions. As research continues, its potential for use in sustainable practices and advanced scientific fields only grows. Understanding its properties, synthesis, and uses is key to leveraging its full potential across various industries.